Abstract
INTRODUCTION Despite the significant improvement in outcomes for patients with multiple myeloma (MM), a proportion of patients with high-risk disease features continue to have relatively poor outcomes. The International Myeloma Working Group defined high-risk cytogenetic abnormalities as the presence of t(4;14), t(14;16), t(14;20), del(17/17p), or gain(1q) in fluorescence in situ hybridization (FISH), nonhyperdiploid karyotype, karyotype del(13) or high-risk signature in gene expression profiling (GEP). There is a concern that a homogenous definition of high-risk MM is not unified in clinical trials. Our study aimed to investigate the high-risk MM definitions in all phase III clinical trials of MM.
METHODS We conducted a cross-sectional analysis of all phase III interventional MM clinical trials registered at Clinicaltrials.gov. We searched the ClinicalTrials.gov database for the terms "myeloma", "multiple myeloma", "plasma cell dyscraisa” and included all studies that are phase III and interventional. Study protocol, data on clinicaltrials.gov and all publications related to the included clinical trials were used for data extraction. Chi-square test was used to study the association between high-risk definition and categorical variables. Multivariable analysis was done using a binary logistics regression model.
RESULTS Our search revealed a total of 327 clinical trials. After screening of all study protocols 52 studies were not related to MM and were excluded. A total of 275 studies were included in our analysis. The definition of high-risk was extracted from the full publication in 88% with the rest of definition obtained from the protocol or data available at clinicaltrials.gov. Only 36% (n=99) of included studies reported the use of at least one definition of high-risk MM. Cytogenetics by either FISH or karyotype were the most used high-risk definition in 94% of studies (n=93/99). Fours studies included the International Staging System (ISS) stage II or III as high-risk while another four studies used GEP in the definition of high-risk. Few studies used laboratory markers solely in high-risk definitions: Beta-2-microglobulin (n=4) and lactate dehydrogenase (n=1). A total of 10% (n=10/99) of included studies used more than one criterion in high-risk definition. The median start date for included clinical trials was 2011 (1998-2021) (IQR: 9) while the median publication date was 2017 (2006-2022) (IQR: 6). Studies that reported a definition of high-risk were those with higher number of enrolled patients, and those with a full publication (p-value <0.001). Location of study (U.S. vs. others) and funding (pharma vs. others) were not associated with higher percentage of high-risk definition.
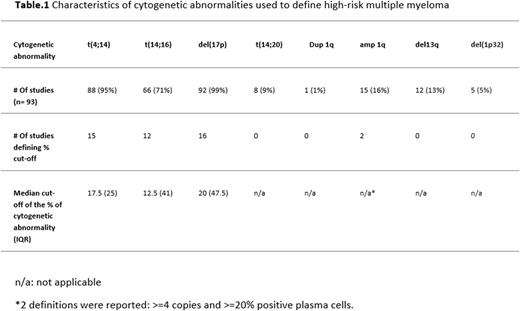
Cytogenetic definition of high-risk included mainly del(17p), t(4;14), or t(14;16) with low number of studies defining a threshold for what is considered to be a positive result and with variable definitions of thresholds in studies that defined a threshold. [Table.1] The use of IMWG definition of high-risk was only followed in 10 studies (10%).
CONCLUSIONS Our analysis indicated that a significant proportion of interventional phase III clinical trials in MM didn't clearly define high-risk MM and when defined the definition used was heterogenous and largely not based on certain thresholds of cytogenetic abnormalities. A unified definition that includes all proven clinical and/or disease high-risk features is needed for future clinical trials.
Disclosures
Schinke:Janssen: Honoraria. van Rhee:GlaxoSmithKline: Consultancy; Karyopharm: Consultancy; Takeda: Consultancy; Janssen Pharmaceuticals: Research Funding; Bristol Myers Squibb: Research Funding; EUSA Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal